Silver Science
Silver Ions
Principles for the antimicrobial properties of silver ions
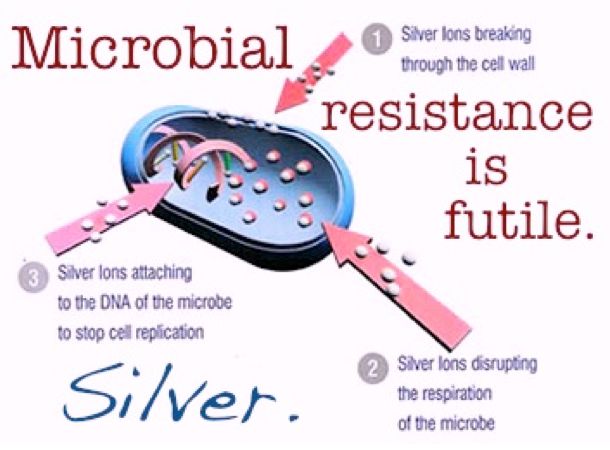
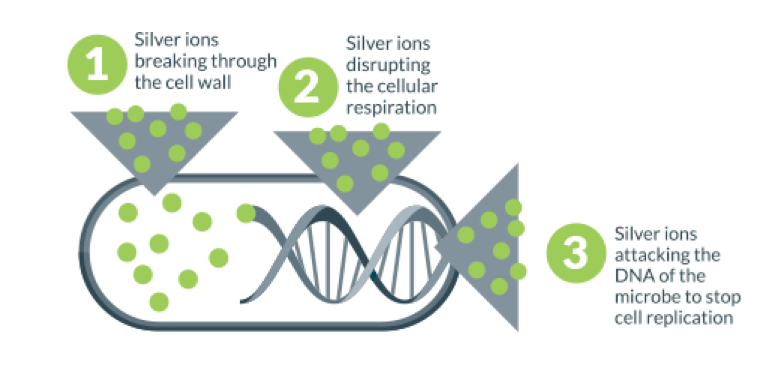
- Positively charged silver ions and negatively charged microbe cells are attracted to each other upon contact.
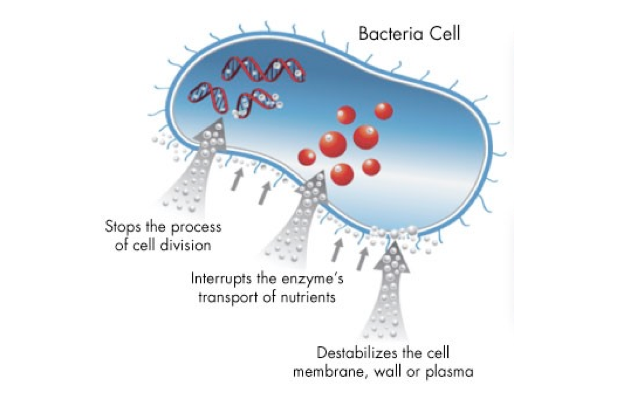
- Silver ions pierce through the external surfaces of the cells and damage their DNA, as well as the protein structure, halting their metabolism and reproduction until they die. This explains the sterilization effect of silver ions.
- After the cells have become inactive, silver ions leave but continue to act against microbes, delivering a constant antimicrobial performance with no toxicity or side effects.
Products protected by ORSi antimicrobial technology will negatively affect bacteria that contaminate the surface through
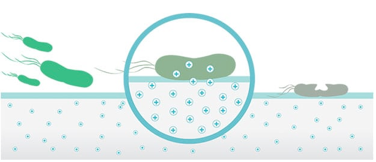
Protein damage
Cell membrane damage
Oxidative damage
DNA Interference
About antibiotic resistance
You can also minimise your own risk of contracting harmful bacteria that could cause an infection by practicing good hand hygiene, preparing and cooking food safely and maintaining a healthy lifestyle.
If we can reduce our chances of catching nasty infections then we will also reduce the need for being treated with antibiotics.
Hence Silver ions can be used to help maintain hygiene standards where keeping harmful Microbes at bay is critical. It’s effective even against drug-resistance bacteria like MRSA and can offer a powerful barrier against Microbes growing on surfaces where they can pose a risk to human health.
| 28 Ni Nickel | 29 Cu Copper | 30 Zn Zinc |
| 46 Pd Palladium | 47 Ag Silver | 48 Cd Cadmium |
| 78 Pt Platinum | 79 Au Gold | 80 Hg Mercury |
Names:
English: Silver
French: Argent
German: Silber
Italian: Argento
Latin: Argentum
Spanish: Plata
Basic Information:
Symbol: Ag
Atomic number: 47
Group number: 11
Mass: 107.868
Density @ 293 K: 10.5 g/cm3
Atomic volume: 10.3 cm3/mol
Melting Point: 961.93 C (1235.1 K)
Boiling Point: 2212 C (2428 K)
Heat of fusion: 11.30 kJ/mol
Heat of vaporization: 250.580 kJ/mol
Number of Protons/Electrons: 47
Number of neutrons: 61
Classification: Transition Metal
Crystal Structure: Face-centered Cubic
Color: silver
Hardness: 3.25 mohs
Characteristics: soft, ductile, tarnishes
Oxidation:
Electron configuration: [Kr] 4d10 5s1
Minimum oxidation number: 0
Maximum oxidation number: 3
Minimum oxidation state: 0 (silver occurs naturally in ores in its elemental state)
Maximum oxidation state: 3 (the unit cell of silver oxide, Ag4O4, has two atoms of univalent silver and two atoms of trivalent silver)
Reactions:
With air: mild, =>Ag2O
With 6M HCl: none
With 6M HCl: none
With 15M HNO3: mild, =>AgNO3
Other Forms:
number of isotopes: 2
hydride(s): none
oxide(s): Ag2O
chloride(s): AgCl
Conductivity:
Thermal conductivity: 429 J/m-sec-degC
Electrical conductivity: 630.5 1/mohm-cm
Electrical resistivity: 1.467 X 10-8 ohms-m (OoC)
Polarizability: 7.9 A^3
Abundance:
Silver occurs in the metallic state, commonly associated with gold, copper, lead, and zinc. It is also found in some 60 minerals including: argentite (a sulfide), cerargyrite (a chloride), many other sulfides and tellurides.
History:
- Uses of silver noted from 4000 B.C. thru this century
- Hippocrates (Father of Medicine) taught about healing power of silver
- Romans used silver in medicine
- Used in other parts of the world for medicinal/healing value of silver
- Unlike anti-biotic medicines silver has practically no bacterial (pathological) resistance
About antibiotic resistance
| Claim | Fact or Fiction | Explanation |
| Need to dump lots of silver metal/ions for anti-microbial efficacy | Fiction | Silver is Oligodynamic – a little goes a long way. Need only 3 – 5ppm to be effective in the media |
| Need good amount of silver to be conductive | Fact | All the silver present need to form a “layer” or layers to be able to allow movement of electrons to be classified as conductive |
| Extruded products with silver are conductive | Fiction | Silver needs to form a solid layer to be able to allow movement of electrons |
| Silver is Thermodynamic | Fiction | From the principles of thermodynamics one can derive general relations between such quantities as coefficients of expansion, compressibility, specific heat capacities, heats of transformation, and magnetic and dielectric coefficients, especially as these are affected by temperature. These are laws not characteristic of a given material |
| Silver is the most Thermally conductive element | Fiction | Silver is quite thermally conductive but not the most. Diamond is the most thermally conductive |
| Silver is most electrically conductive element | Fact | It is the more conductive than Gold |
| Silver is most malleable and ductile element | Fact | Hence it is so easy to use in everyday life |
| Silver is effective against Fungi | Fact | It is very effective against fungi such as yeasts and molds |
| Silver is broad spectrum anti-bacterial element | Fact | It is very effective against both Gram Positive and Gram Negative bacteria |
| Silver is most reflective element | Fact | Silver mirror are best example of this. This property helps in providing good comfort for wearers of silver products both in hot and cold conditions |
| Silver has good IR signature | Fact |
Anti-microbial pathway
- Protein denaturation
- Blocks respiratory chain
- Metabolite efflux
- Blocks DNA replication
- Massive proton leakage
Anti-odor
- Bonding of denatured protein of dead bacteria
- Chemical bonding of other odor causing agents
Anti-Static
- As in anti-microbial property; a little of Ag Flex goes a long way.
- Either as grounded delivery system or as corona discharge pathway Ag Flex moves charge by conducting it through the entire pathway to eventually into the ground or distributing the charge so that it will be spread evenly and hence make the article safe.
Thermal properties
- Due to conductive nature of silver; energy in form of heat is evenly distributed across the article incorporated with Ag Flex.
- This enables movement of heat resulting in keeping the subject cooler when it is warm or warmer when it is cold.
- Measurement is done for thermal conductivity as CLO value or thermal conductivity. It is also measured in terms of “Comfort Factor”.
